Vordiplom, Universität of Münster, Germany, 1988
Ph.D. UMass, Amherst, 1993
Postdoc. Harvard Medical School, 1994-1998
Keywords: protein transport, proteomics, post-translational modifications, glycosylation, type IV pili, flagella, S-layer, biofilm formation, archaea
Prokaryotic cell surface biogenesis and function
Bacteria and archaea have complex cell envelopes that have several important functions, including providing a barrier that protects the cytoplasm from the environment. Along with its associated proteinaceous structures, it also ensures cell stability, facilitates motility and mediates adherence to biotic and abiotic surfaces. Although archaea are ubiquitous, present in all habitats examined thus far, including the human microbiome, compared to the bacteria, little is yet known about this domain of life. Using the genetically and biochemically amenable model archaeon Haloferax volcanii, my lab employs well-established techniques of molecular biology, biochemistry, and microscopy, as well as cutting edge technologies, such as proteomics, to characterize the unique archaeal, as well as the highly conserved, aspects of archaeal cell surface biology.
Our lab’s extensive characterization of the evolutionarily conserved Sec pathway, as well as the Tat pathway, advanced the understanding of protein transport across both domains of prokaryotes. Exciting results from our recent analyses of cell surface anchoring strategies of archaeal proteins have uncovered a seemingly unique archaeal surface anchoring strategy and also revealed a novel membrane anchoring mechanism that is conserved between archaea and bacteria. Furthermore, our analyses of archaeal surface filaments led to the identification of previously unknown roles of type IV pilins and glycosylation in the regulation of early steps in biofilm formation.
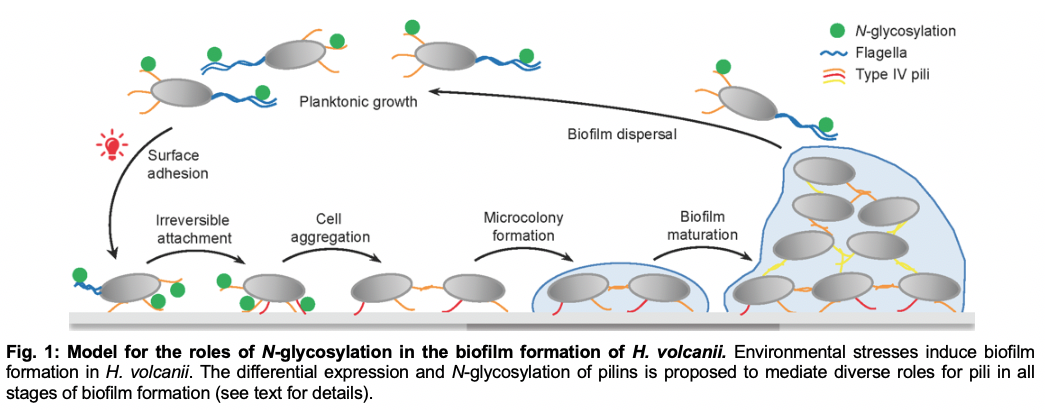
The lab has used the data gleaned from these in vivo studies to develop novel software programs, as well as to improve existing ones, that predict the substrates that use specific transport and cell surface anchoring pathways. Moreover, our lab recently spearheaded the Archaeal Proteome Project, to advance the knowledge of archaeal cell biology through comprehensive proteomics. Information gleaned from these studies have also enhanced our understanding of bacterial cell surface biogenesis and function and the evolution of the three domains of life (see FEMS review and the recent PennToday article).
BIOL 375 - Microbial Diversity and Pathogenesis
BIOL 376 - Microbial Divesity and Pathogenesis Lab
Click here or on PubMed link

